Abstract
Anemia of inflammation is characterized by low hemoglobin and normal/high total body iron. This condition is driven by pathologic elevations in hepcidin, a central regulator of iron homeostasis. DISC-0974 is a first-in-class monoclonal antibody in development for anemia of inflammation that blocks hemojuvelin (HJV), a co-receptor in the BMP signaling pathway driving hepcidin expression. Preclinical studies in animal models with inflammatory stimuli have shown that DISC-0974 suppresses hepcidin and increases serum iron. In this first-in-human, Phase 1a, double-blind, placebo-controlled single-ascending dose study, we assessed the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of DISC-0974 in healthy volunteers.
Eligible participants included healthy males and females of non-reproductive potential, ages 18 to 65 years old. Dose escalation proceeded through 7 mg IV, 14 mg SC, 28 mg SC and 56 mg SC; a 28 mg IV cohort was then evaluated for IV and SC comparability. Treatment was allocated in a 3:1 ratio with placebo, with 8 participants total planned per cohort. Two additional replacement participants were enrolled in the 7 mg IV cohort and are included in the analysis. Dose escalation stopping rules were met in the 56 mg SC cohort.
Primary endpoints included adverse events (AEs), clinical laboratory assessments, vital signs, physical examination, and electrocardiograms. Secondary endpoints were standard PK parameters and PD metrics including serum hepcidin-25, iron, transferrin saturation (TSAT), and hematology biomarkers. Samples were analyzed for DISC-0974 concentrations using a validated electrochemiluminescence immunoassay method. Serum hepcidin was analyzed using a validated ELISA method (IDx).
Safety, PK, and PD data were summarized using descriptive statistics. Multivariate regression modeling was performed to identify baseline characteristics associated with PD responses.
A total of 42 participants were enrolled. Demographics and baseline characteristics showed a generally balanced representation of age, gender, ethnicity, race, height, and weight. There were no serious AEs (SAEs), Grade 2 or higher AEs, or AEs leading to study withdrawal. Two Grade 1, non-serious AEs were considered possibly related to study drug, as they were reported within 24 hours of dosing; they resolved shortly after onset without medical intervention. No clinically significant safety laboratory findings were observed, including no elevations in liver function tests.
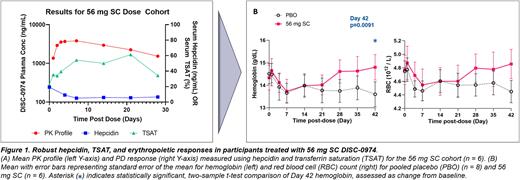
DISC-0974 PK profiles were consistent with anticipated patterns for IV and SC dosing routes. PD analysis showed that DISC-0974 treatment led to dose-dependent hepcidin suppression and increased serum iron. The most robust iron response was observed in the 56 mg SC group, with sustained iron and TSAT elevation for 3 weeks post-treatment and return to baseline by 28 to 35 days post-dosing. Within the same time frame, ferritin levels showed decreasing trends. Reticulocyte and RBC hemoglobin content showed increasing trends. Absolute reticulocyte count also showed increasing trends. Overall, these findings indicated enhanced mobilization of iron stores into maturing erythrocytes. By 42 days post-treatment, hemoglobin levels and RBC counts were higher in the 56 mg SC subjects as compared to placebo controls. In addition to treatment group, multivariate regression modeling showed that baseline ferritin was a key predictor of TSAT response. A backward stepwise regression modeling approach also identified baseline age, mean corpuscular volume, and reticulocyte hemoglobin as modifiers of the TSAT response. Ethnicity, race, gender, weight, and baseline hepcidin were not significant.
In summary, the study results allowed defining a useful range of DISC-0974 dosing that supports a favorable safety/tolerability profile and clinically relevant levels of hepcidin suppression, serum iron elevation, iron store mobilization into erythrocytes, and total hemoglobin and red blood cell count increases. These findings support the development of DISC-0974 as a potentially clinically meaningful treatment for anemia of inflammation. A phase 1b clinical trial in patients with myelofibrosis and anemia with monthly dosing of DISC-0974 is currently ongoing.
Disclosures
Novikov:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Buch:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Yang:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Tuller:Disc Medicine: Current holder of stock options in a privately-held company, Ended employment in the past 24 months. Andruk:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Chan:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Wu:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Rodriguez:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Panwar:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Howell:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. MacDonald:Disc Medicine: Consultancy, Current equity holder in private company, Current holder of stock options in a privately-held company. Savage:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal